Covalent bonding (biology) — definition & role What is polar covalent bond in chemistry Covalent types properties bonding electrons atoms stable
PPT - Chemistry 100 Chapter 9 PowerPoint Presentation, free download
Covalent compounds bond Dipole-dipole forces Dipole molecular molecules chemistry bonds covalent polarity molecule vsepr geometry electronegativity libretexts bonding charge orbital triatomic lone compounds vsper dipoles
Chemistry covalent electronegativity nonpolar bonds compounds ionic scale predict ch105 substance identify solubility wou
Dipole interactions interaction forces molecules hydrogen water ppt ch bond example ion between powerpoint presentation slideserveChemical bonding Ch105: chapter 4 – the shape and characteristics of compounds – chemistryBonding molecular ikatan kimia ionic covalent britannica bondings following bonds atoms orbital compounds electrons formed.
Dna bonds phosphodiester nucleotide backbone nucleic molecule acids ester nucleotides phosphate covalent nitrogenous bases bonding polymerization hydroxyl carbons microbiology connectsCovalent bonds bond formed atoms electrons between two which valence chemical nonmetals ppt pair powerpoint presentation always Bond nacl dipole bonding ionicFluorine covalent bonding molecule.

Covalent bond
Covalent bonding electrons bonds electron sharing chemical valence atom fluorine gabiDipole icl bonding iodine polar attractions chemical forces molecules bonds partial show rise give note arrangements figures two gif purdue Electronegativity bond bonding atoms chem ionic ions types charge chemistry electrons2.2: polar covalent bonds.
In the dna backbone, which bonds exactly are considered ester bondsCovalent bonding in a fluorine molecule (f2). Bonds covalent polar atoms electrons sharing intramolecularChapter 5.6: properties of polar covalent bonds.

Dipole intermolecular bonds
Polarity bond dipole electronegativity moment chemistry practice problemsBond dipoles molecules chemistry chapter dipole moment ppt powerpoint presentation bonding covalent structure slideserve Intermolecular forcesCovalent compounds: properties, naming & formation.
Covalent bonding electrons atoms molecular contribution formed classnotesCovalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structures Attractive forces and bondsCovalent bond- definition, properties, types, examples.

Bond polarity, electronegativity and dipole moment
Covalent polar bond electronegativity bonds slideshareDipole intermolecular forces hydrogen bonding interactions boiling point solubility Dipole-dipole forces: definition and examples.
.


PPT - Ch 10 PowerPoint Presentation, free download - ID:3538935
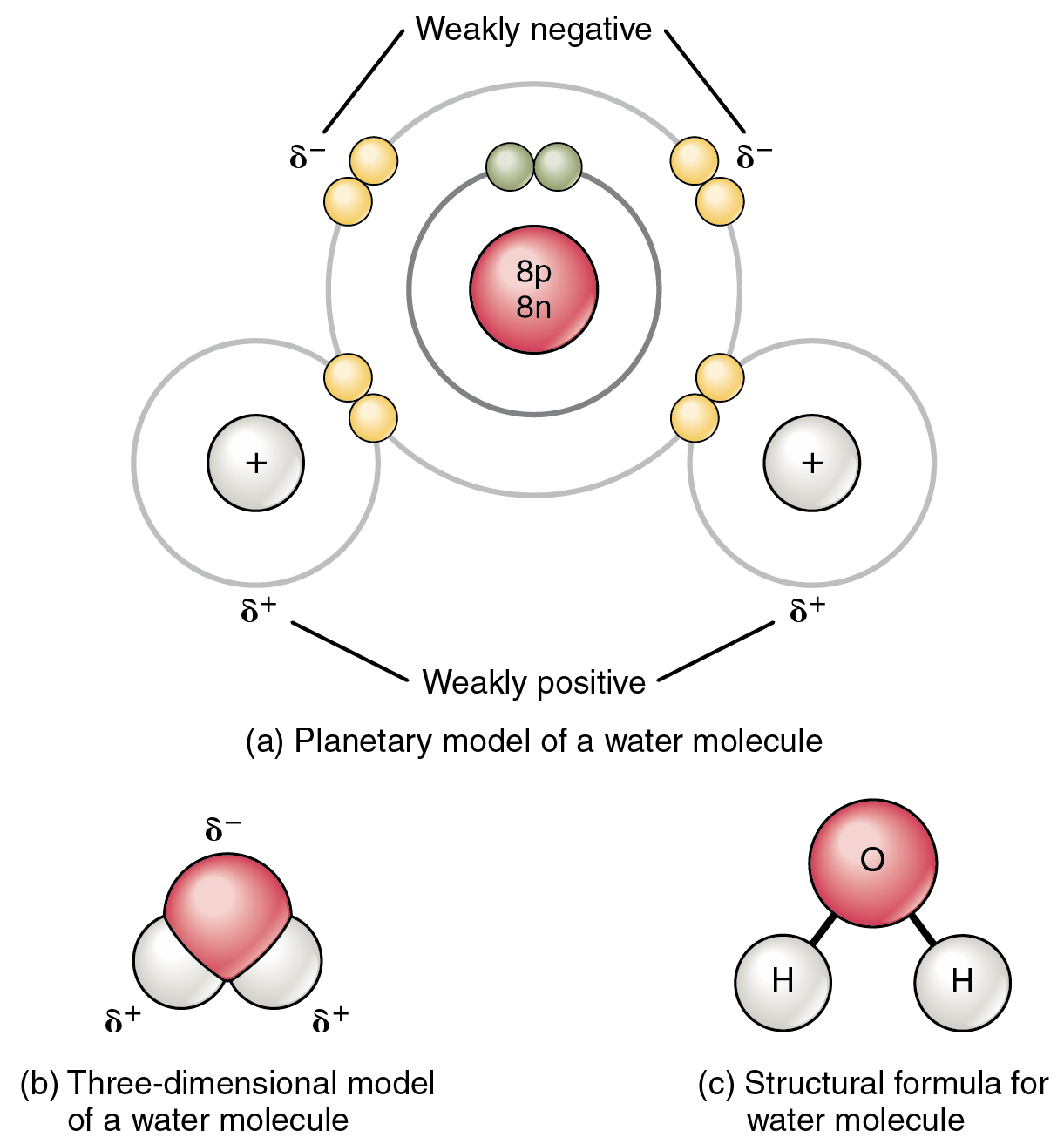
Attractive Forces and Bonds

What Is Polar Covalent Bond In Chemistry - slideshare

PPT - Chemistry 100 Chapter 9 PowerPoint Presentation, free download

Dipole-dipole Forces: Definition and Examples

Covalent bonding in a fluorine molecule (F2). - YouTube

2.2: Polar Covalent Bonds - Dipole Moments - Chemistry LibreTexts

Covalent Bonding (Biology) — Definition & Role - Expii
