How is a single covalent bond formed? + example Covalent bond polar bonds chemistrylearner Covalent molecules compounds chemistry bonds molecular elements introduction
Double Covalent Bond | Facts, Definition, History & Examples
Covalent compounds naming metals How are covalent bonds formed Covalent bonds chlorine atoms electrons electron forming monahan expii
Bonds covalent ionic formed electrons
Covalent bond ionic bonding bonds electrons formation formed chemistry atoms differencesCovalent bond formulas bonding acids including names go ppt powerpoint presentation Ionic bonds, polar covalent bonds, and nonpolar covalent bondsChapter 5.6: properties of polar covalent bonds.
Double covalent bondBonds covalent compounds carbons pairs bond electron hydrogen molecule formed Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.

Covalent polar bonds bond ionic bonding libretexts electron structures atoms electrons nonpolar molecular purely
Covalent vs ionic bond- definition, 11 key differences, examplesCovalent naming compounds rules nomenclature Covalent bond — formation & compoundsCovalent bond: definition, types, and examples.
Naming covalent compoundsIonic covalent polar nonpolar bonds Ch150: chapter 4 – covalent bonds and molecular compounds – chemistrySingle bond covalent formed bonds example lines represented.

Bond covalent double carbon examples two definition formation atoms between forms ethene shown figure dioxide
Covalent bonds compounds chemistry ionic molecular vs bond chapter atoms molecule figure their hydrogen intoMultiple bonds — double & triple bonds Covalent bonds ionic metallic between formed difference examples bond covalency oxidation state vs compounds hydrogen definition properties molecules pediaaCovalent bond bonds ionic formed between difference vs types examples atoms properties definition.
How is a covalent bond formed?Periodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular has symbols dot columns .


Covalent vs Ionic Bond- Definition, 11 Key Differences, Examples

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

PPT - Covalent Bonding Formulas & Names (including Acids) PowerPoint

PPT - Ionic and Covalent Bonds PowerPoint Presentation, free download
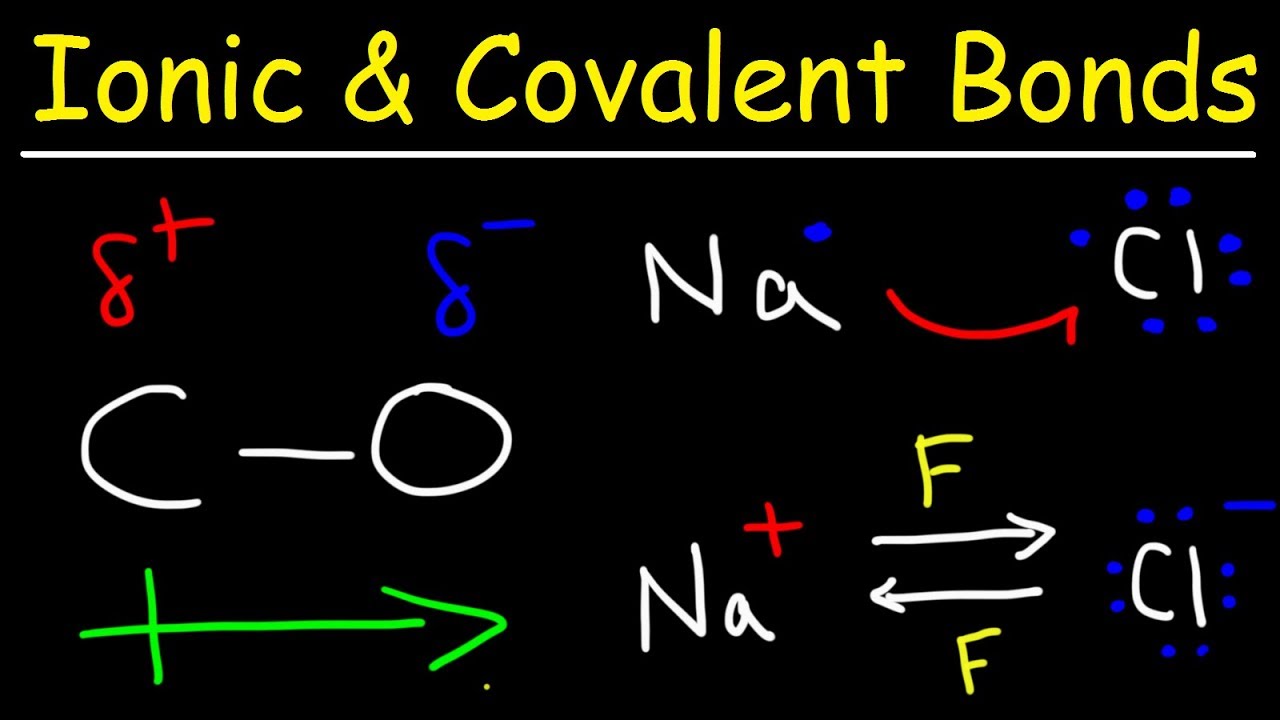
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds - YouTube
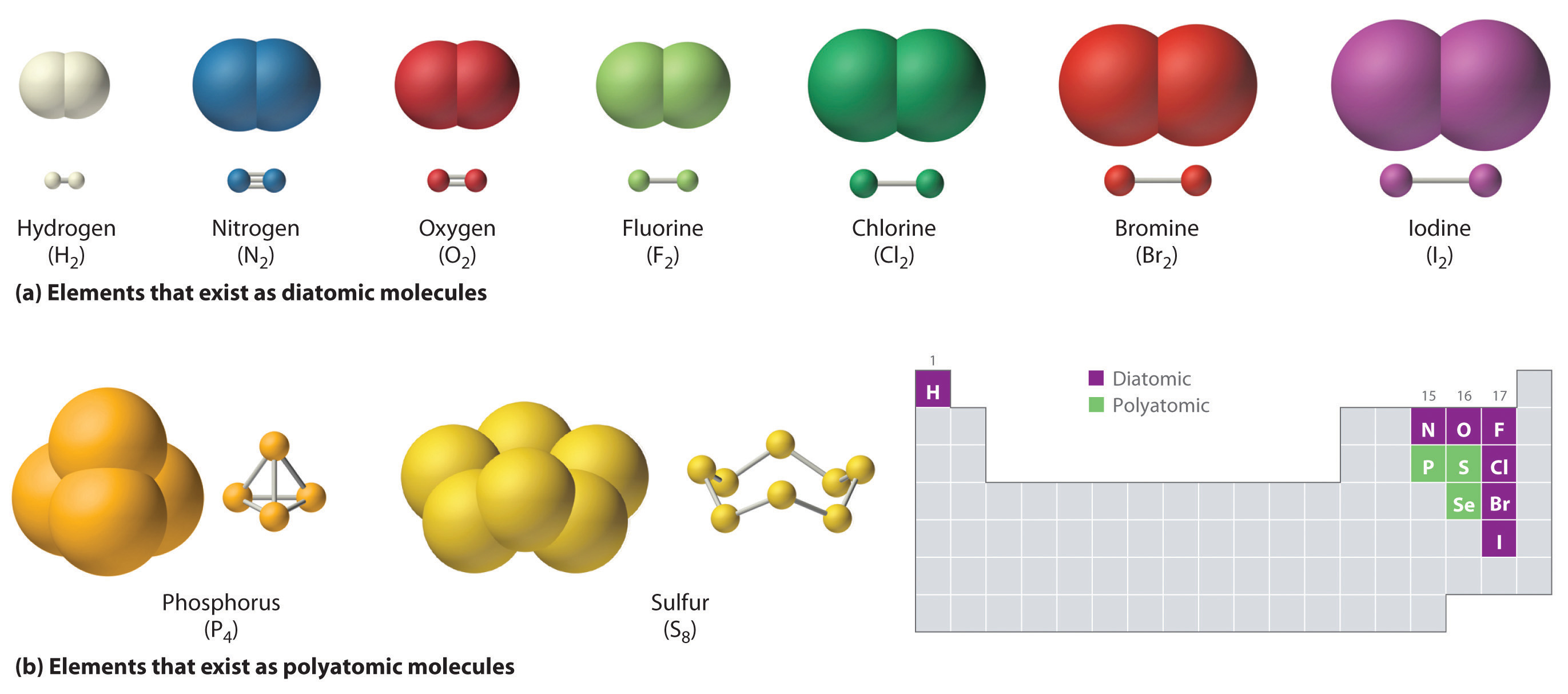
CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

PPT - Covalent Compounds and Naming PowerPoint Presentation, free

Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts

How is a single covalent bond formed? + Example
